.png)
In the pharmaceutical industry, sterilization is one of the most critical processes for ensuring the safety, quality, and efficacy of drug products. It is the validated process of eliminating or killing all viable microorganisms, including bacteria, fungi, and spores, from pharmaceutical products, packaging, and equipment. Whether it's injectables, ophthalmic solutions, or surgical tools, sterilization is essential to safeguard patients and comply with stringent regulatory requirements.
Patient Safety: Sterilization guarantees that pharmaceutical products are free from microbial contamination. This is especially vital for injectable and ophthalmic drugs, where even minor contamination could lead to severe infections and health risks.
Product Quality: Microbial contamination can alter the composition, stability, or potency of a drug. By ensuring sterility, manufacturers preserve the intended therapeutic effectiveness of their products.
Regulatory Compliance: Regulatory bodies such as the FDA, EMA, and WHO mandate validated sterilization processes for pharmaceutical manufacturers. Meeting these standards not only ensures safe usage but also strengthens brand credibility.
Moist Heat (Steam) Sterilization: The most widely used technique, moist heat sterilization, employs high-pressure steam in an autoclave. It is suitable for heat-stable products like surgical dressings, instruments, and containers.
Dry Heat Sterilization: This method involves exposing products to high temperatures in a convection oven. It is ideal for moisture-sensitive substances like dry powders, oils, and oily injections.
Filtration Sterilization: For heat-sensitive solutions, such as intravenous drugs and ophthalmic preparations, sterile filtration removes microorganisms using fine-pore filters without damaging the product.
Chemical Sterilization:
Irradiation (Radiation) Sterilization: Using gamma rays or electron beams, this method disrupts microbial DNA. It is effective for certain drugs and packaging but requires compatibility checks to ensure product stability.
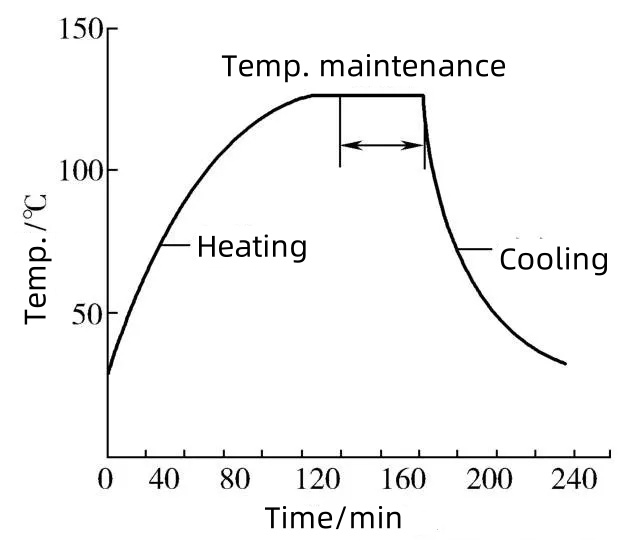
Thermal sterilization is one of the most validated and reliable sterilization techniques used in the pharmaceutical industry, particularly for heat-stable products. Key values include:
This is applicable for an expansion of a single cell i.e., no clogging of cells.
Logarithm of the variable cell concentration percentage(fraction) is proportional to the time of exposure at the (high) temperature.
Such relationship results when the rate of decreases in variable cell concentration is directly proportional to the variable cell concentration present at any time.
Let we see how it happens:
Rate of decrease in concentration ∝ Xv
rd ∝ kd Xv
Let us write a balance on cell taking the bioreactor froth as the system,
Taking Mass Balance,
( rate of cells i/p in froth ) - ( rate of cells o/p froth) + ( rate of generation of cell in froth ) - ( rate of conduction of cell in froth) = ( accumulation of cells in feoriactor froth)
Thus,
Thus,
Since consumption of cells through death
Because Rd is on concentration base and rc is on a mass base
From The definition of concentration
Mx = Xv V
-rd v = d(Xv V)/dt = V d(Xv)/dt
∴ -rd = -kd Xv = d(Xv)/dt
If we first solve this first order differential equation,
We get,
\[ \ln\left(\frac{x_{v0}}{x_v}\right) = k_d t \]
The time needed for the available cell concentration to go down to Xv starting from Xv0 is
\[ t = \frac{2.303}{k_d} \log_{10} \left( \frac{x_{v0}}{x_v} \right) \]
At Meknetics, we understand the complexity and importance of sterilization in the pharmaceutical industry. With expertise in material handling systems, automation, and sterile equipment solutions, we help manufacturers achieve uncompromising standards of safety and quality. Whether you are dealing with heat-sensitive formulations, moisture-restricted products, or large-scale equipment sterilization, our solutions are designed for efficiency, compliance, and reliability.
Get in touch with Meknetics today at 0484-3532826 or 📧 info@meknetics.com. Explore our services at https://meknetics.com/ and take the first step toward safer, compliant, and more efficient pharmaceutical processes.
Meknetics Innovative Solutions
Posted: 30 September, 2025